investors
ECI exists at the intersection between hematology/transfusion medicine and multiple medical specialties ranging from ophthalmology to wound care.
We are focused on a development pipeline that includes drug candidates that will meet critical needs and alleviate suffering, and we’re glad you’re partnering with us to make these advances a reality.
ECI has been issued robust patents protecting the formulation that combines serum-like plasma with a novel antimicrobial molecule (chitosan) to create products ranging from liquid eye drops to healing gels, ointments, contact lenses, contoured bandages and wound dressings.

eci background
ECI’s initial focus is on the anterior segment of the eye, including neurotrophic keratitis (NK), and dry eye disease (DED).
Our team, comprised of experts in ophthalmology, human plasma, and pathogen safety, has successfully brought multiple ophthalmic and blood-derived products and drugs to market. Leveraging that experience, we are dedicated to delivering this transformative technology, positioning us for success in the ophthalmic treatment landscape before expanding into adjacent fields.
- 4 issued, 5 pending patents
- established regulatory pathway
- supply chain established
- manufacturing partner in place
investment highlights


the need
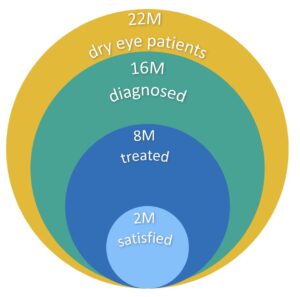
- NK: 5 in 10,000 patients in the US
- DED: 22 million dry eye patients in the US alone
- 500 million adults with diabetes at risk of hard-to-heal wounds
- ~10% of individuals and 25% of older people experience dry mouth
- >50% of postmenopausal individuals experience gynecologic dryness

the market
- $1.5 billion NK (US market)
- $4 billion dry eye (US market)
- $24.5 billion wound care
- $600 million dry mouth
- $32 million gynecologic dryness


dry eye disease

dry eye disease
Key trends contributing to growth
- ↑ aging population
- ↑ autoimmune disorders
- ↑ computers smartphones
- ↑ warmer, drier weather
Treatments: Dry eye is caused by several conditions. Primary treatment is using an eye drop. However, side effects caused by eye drops, such as blurred vision, increased sensitivity, watering eyes, redness, swelling of eyelids, sticky eyelashes, and discomfort decrease adoption.
Farrand KF et al. AJO (2017), Dubey VD et al. IOVS (2010), Lum et al. AA of Optometry (2018)
ECI believes it is likely that serum eye drops will have fewer side effects. This belief is based on experience with autologous serum drops.
global dry eye treatment market size and projected growth
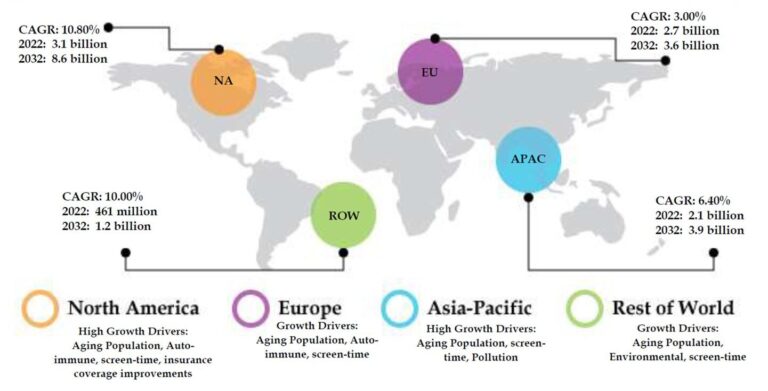
Coherent Market Insights, July 2020

learn more about:
our products
Our therapeutic formulation is suitable for a variety of products, including liquid eye drops, gels, ointments, contact lenses, contoured bandages, and wound dressings. Initially, ECI is pursuing ophthalmic indications for neurotrophic keratopathy (NK) and dry eye disease (DED).
ECI’s pipeline includes indications for ophthalmic burns, epithelial defects, endothelial diseases, limb stem cell deficiency, ocular graft-versus-host disease (GvHD), corneal abrasions, and chronic wounds.
our platform
Our platform leverages growth factors derived from donated human platelet-rich plasma and proprietary hyaluronic acid (HA) or chitosan. The tissue-repairing benefits of plasma-derived products, combined with the sustained action and impact of HA or chitosan, may provide relief and long-lasting therapeutic action.
our team
Our team is comprised of experts in hematology, ophthalmology, biomedical/biomaterial engineering, quality/ regulatory affairs, project management, drug design, pre-clinical trials, and device development.
We like dogs, hiking, swimming, sailing, making sushi, espresso, baking, and reading. And eyes, blood, and science, of course!