neurotrophic keratitis & dry eye disease
Neurotrophic keratitis (NK) is a rare, degenerative corneal disease characterized by reduced or complete loss of corneal sensation, leading to impaired wound healing and corneal damage. NK often arises from conditions such as herpes simplex or zoster infections, diabetes, or surgical damage to the trigeminal nerve.
NK is classified into stages ranging from epithelial defects to ulceration and perforation of the cornea, which, if left untreated, can lead to vision loss. Growth factors play a critical role in the treatment of NK by promoting nerve regeneration and corneal healing. These biologically active proteins stimulate cell proliferation, repair damaged nerves, and restore the structural and functional integrity of the cornea, offering a targeted therapeutic approach for this challenging condition. ECI’s biologically-derived growth factor formulation helps address NK patients’ unmet needs.
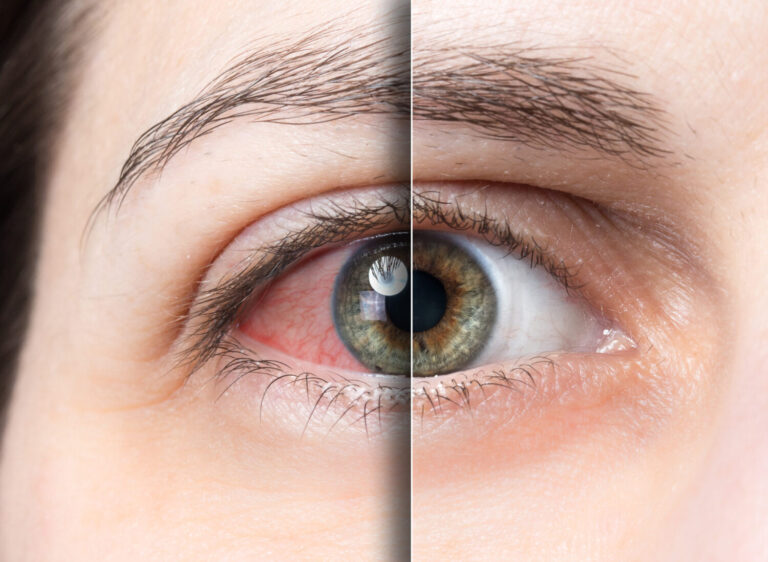
Dry eye disease (DED) symptoms, such as dryness, burning, itching, and redness, can significantly affect a person’s quality of life, making it difficult to perform everyday activities, such as reading, using a computer, or driving. In some cases, DED can also lead to complications, such as corneal abrasions or infections, which can cause further discomfort and vision problems. The chronic nature of DED can even lead to feelings of frustration, anxiety, and depression.
did you know...
Dry eye disease can be a debilitating condition?
It’s true, particularly if left untreated or undertreated. The severity of dry eye disease can vary greatly, and some people may have mild or intermittent symptoms that do not significantly impact their daily activities but for others it’s devastating! This depends on their symptoms and the effectiveness of their treatment.
plasma-derived serum eye drops
Our initial two products will focus on neurotrophic keratitis (NK) and dry eye disease (DED).
5 in 10,000 people in the United States have NK. This makes it a rare disease and creates the potential for orphan drug status.
There are 22 million dry eye disease patients in the United States, but only 8 million are treated, and 25% report satisfaction with their treatment.
There is a need for more effective treatments with fewer side effects for both NK and DED.

learn more about:
our products
Our therapeutic formulation is suitable for a variety of products, including liquid eye drops, gels, ointments, contact lenses, contoured bandages, and wound dressings. Initially, ECI is pursuing ophthalmic indications for neurotrophic keratopathy (NK) and dry eye disease (DED).
ECI’s pipeline includes indications for ophthalmic burns, epithelial defects, endothelial diseases, limb stem cell deficiency, ocular graft-versus-host disease (GvHD), corneal abrasions, and chronic wounds.
our platform
Our platform leverages growth factors derived from donated human platelet-rich plasma and proprietary hyaluronic acid (HA) or chitosan. The tissue-repairing benefits of plasma-derived products, combined with the sustained action and impact of HA or chitosan, may provide relief and long-lasting therapeutic action.
our platform
product safety
The safety of our products is paramount. To ensure that our products are as safe as possible, pathogen inactivation and removal technologies like the INTERCEPT Blood System for Plasma and nanofiltration have been incorporated into our manufacturing process.
ECI has met with the FDA and aligned on an orthogonal (multi-step) safety plan meeting all regulatory requirements.