platelet-rich plasma-derived growth factor eye drops
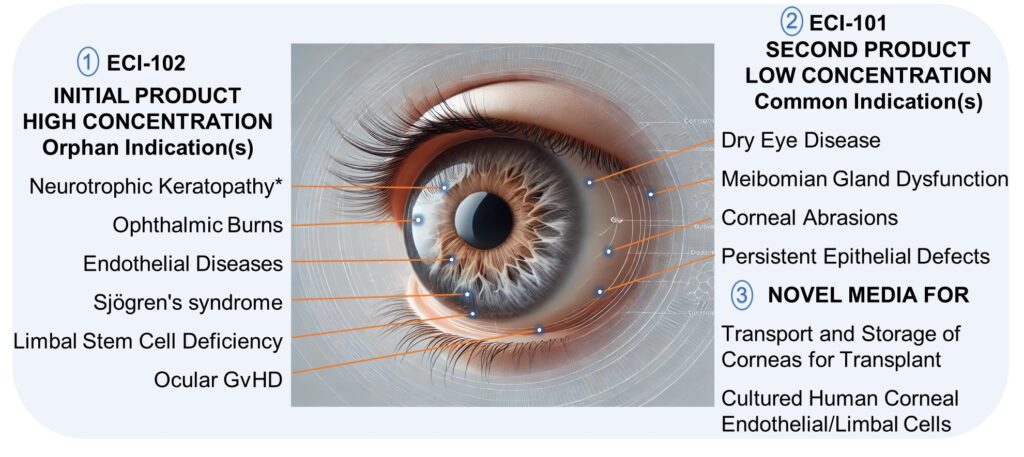
ECI-102 and ECI-101 are ECI’s novel platelet-rich plasma-derived growth factor eye drops. These drops are formulated to provide accelerated healing of persistent epithelial defects on the eye. The first two products in ECI’s pipeline are ECI-102, for treating neurotrophic keratitis, an orphan disease, and ECI-101, for treating dry eye disease and corneal abrasions.
ECI-102 contains an increased concentration of growth factors, while ECI-101 contains our standard concentration of growth factors.

Doctors caring for patients with ocular surface disease refractory to artificial tears and topical medications have turned to platelet-rich plasma and serum eye drops. Platelet-rich plasma eye drops are a near-physiologic replacement for the natural tear and include the supportive and protective factors that naturally occur in healthy human tears. They contain similar proteins, electrolytes, and antimicrobial compounds found in healthy tears.
The advantage of platelet-rich plasma is that many of its biochemical characteristics, including pH, osmolality, vitamins, fibronectin, and growth factors are similar to human tears. In vitro and in vivo studies have demonstrated that treatments enhance corneal epithelial care, inhibit the release of inflammatory cytokines, and increase the number of goblet cells and mucin expression in the conjunctiva.
Platelet-rich plasma drops have been shown to contain anti-inflammatory properties not only by containing inhibitors of inflammatory cytokines, but they also increase goblet cells that produce the gel-forming secretory mucin MUC5AC. Platelet rich plasma has also been shown to increase expression of all the membrane-associated mucins in the human ocular surface, MUC1, MUC4, and MUC16, in HCjE telomerase-immortalized human conjunctival epithelial cell lines.
ECI is enabling access to this proven API by developing allogeneic growth factor concentrates.

overcoming risks and challenges
Platelet-rich plasma derived eye drops are known to be efficacious, but today these products are made from a patient’s own blood, not fda approved, not sterilized, and difficult to obtain.
ECI will deliver eye drops manufactured from safe donated human plasma that can provide these benefits without individuals needing to undergo repeated needle sticks and blood draws. Plasma used for ECI’s production will undergo safety testing and safety procedures beyond those currently performed on transfused blood products within the USA. ECI’s plasma will undergo multiple pathogen inactivation steps and be enriched with hyaluronic acid (improving viscosity with healing properties).
Unlike existing platelet-rich plasma or serum drops, made from a patient’s own blood, ECI’s eye drop will be commercially manufactured, FDA-approved, and broadly available. It will contain vital components not found in other artificial tears or dry eye treatment products.

learn more about:
our platform
Our platform leverages growth factors derived from donated human platelet-rich plasma and proprietary hyaluronic acid (HA) or chitosan. The tissue-repairing benefits of plasma-derived products, combined with the sustained action and impact of HA or chitosan, may provide relief and long-lasting therapeutic action.
our patients
Patients who experience conditions ranging from neurotrophic keratitis (NK) to dry eye disease (DED), wounds, or burns may benefit from our fusion of plasma and HA as a forward-thinking and potent strategy to potentially address their conditions with easier access and fewer side effects.
product safety
The safety of our products is paramount. To ensure that our products are as safe as possible, pathogen inactivation and removal technologies like the INTERCEPT Blood System for Plasma and nanofiltration have been incorporated into our manufacturing process.
ECI has met with the FDA and aligned on an orthogonal (multi-step) safety plan meeting all regulatory requirements.