product safety
Several drugs and biologics (including ECI-101 and ECI-102) are manufactured from human plasma. They have been used for decades in treating bleeding disorders, autoimmune and inflammatory diseases, immune deficiency, and more.

The safety of our products is paramount. To ensure that our products are free from pathogens, innovative technologies like the INTERCEPT™ Blood System for Plasma and nanofiltration are integrated into our manufacturing process.
pathogen reduction of human plasma


Safety step 1: INTERCEPT™ Blood System for Plasma
why pathogen reduction?
- safeguards our products
- enables product manufacturing from donated plasma
- leverages FDA-approved methods
- sets ECI apart
Safety step 1: The INTERCEPT Blood System for Plasma uses a process called pathogen inactivation. This technique employs the use of amotosalen, a photosensitive molecule, which, when exposed to ultraviolet (UV) light, forms covalent bonds with the nucleic acids of pathogens, effectively inactivating them. By preventing these pathogens from replicating, the risk of transfusion-transmitted infections is reduced. This process is especially effective against a wide range of viruses, bacteria, and parasites that might be present in the plasma.


nanofiltration
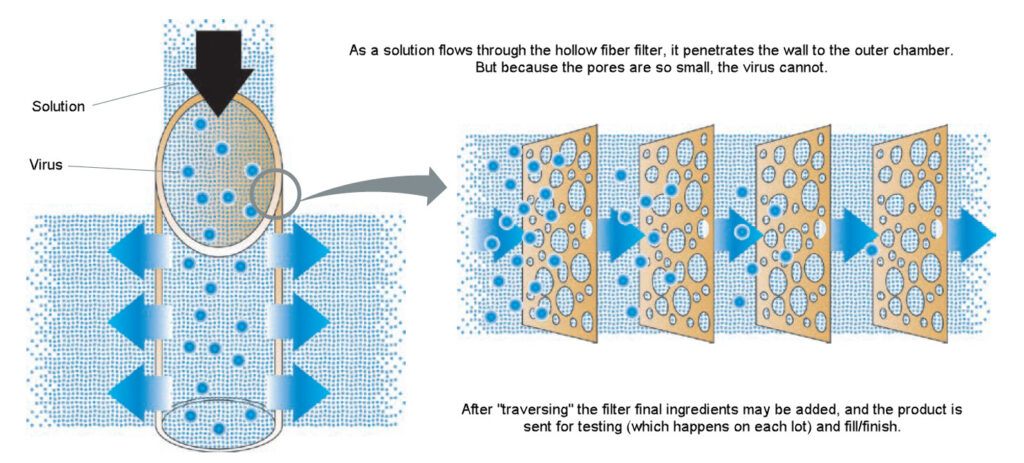

Safety step 2: Nanofiltration
Safety step 2: Nanofiltration is a physical process that involves passing our product through filters with pores in the 20 nanometer size range. For comparison, a hair is 50,000 to 100,000 nanometers in diameter! These ultra-small pores are specifically designed to trap and remove viruses based on their size, irrespective of their ability to replicate or their resistance to chemical inactivation. By doing so, nanofiltration provides an additional layer of safety by catching pathogens that might have escaped or are resistant to chemical treatments.
When combined, the INTERCEPT system and nanofiltration offer a comprehensive approach to blood safety. While the INTERCEPT system targets the nucleic acids of pathogens, ensuring they can’t replicate, nanofiltration acts as a secondary barrier, physically removing any potential residual contaminants based on size. The use of both these technologies in tandem can produce a near-sterile blood product, further ensuring the safety of recipients.


learn more about:
human plasma
Platelet-rich plasma is high in growth factors and proteins. These components are known to promote cell proliferation, tissue regeneration, and repair, making them beneficial for mucosal health. Additionally, plasma’s inherent composition naturally hydrates dry mucosal membranes.
our products
Our therapeutic formulation is suitable for a variety of products, including liquid eye drops, gels, ointments, contact lenses, contoured bandages, and wound dressings. Initially, ECI is pursuing ophthalmic indications for neurotrophic keratopathy (NK) and dry eye disease (DED).
ECI’s pipeline includes indications for ophthalmic burns, epithelial defects, endothelial diseases, limb stem cell deficiency, ocular graft-versus-host disease (GvHD), corneal abrasions, and chronic wounds.
our platform
Our platform leverages growth factors derived from donated human platelet-rich plasma and proprietary hyaluronic acid (HA) or chitosan. The tissue-repairing benefits of plasma-derived products, combined with the sustained action and impact of HA or chitosan, may provide relief and long-lasting therapeutic action.
INTERCEPT™ is a registered trademark of Cerus Corporation, Concord, CA